Draw The Electron Configuration For A Neutral Atom Of Zinc
Draw The Electron Configuration For A Neutral Atom Of Zinc - The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. Draw an orbital diagram and use it to derive the electron. Web now in the next step, start drawing the orbital diagram for zinc. Hence, draw the blank orbital diagram of zinc up to 3d subshell as follows: The transition metals still do not end up being isoelectronic with a. Web the atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. The fact that the electron configuration shows that all sublevels are full, indicates that there are no unpaired electrons. According to the electron configuration chart, electrons in an atom occupy orbitals according to their increasing energy, with each orbital having a maximum of two paired electrons with opposite spins. Web if we lose two electrons, we have a net deposited two charge. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We first need to find the nu. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. Web electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as full. Electron configuration of gallium (ga) [ar] 3d 10 4s 2 4p 1: The electron configuration for calcium two plus would be the same as the electron configuration for the noble gas argon here. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas. Web therefore, the number of electrons in neutral atom of zinc is 30. The electron configuration for zn +2: When writing an electron configuration, you have to write serially. 1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10. H #1s^1# he #1s^2# li #1s^2 2s^1# be #1s^2 2s^2# b #1s^2 2s^2 2p^1# c #1s^2 2s^2 2p^2# n #1s^2 2s^2 2p^3# o #1s^2. The two electrons that we would lose to form the calcium two plus ion are these. The ground state electron configuration of zn is [ar] 3d 10 4s 2.the. The abbreviated electron configuration of zinc is [ar] 3d 10 4s 2. This indicates that zinc has the same electronic structure as the noble gas argon (ar), followed by two electrons in the 4s orbital and ten electrons in the 3d orbital. Web the atomic number of zinc is 30, which means that all zinc atoms have 30 protons in. Write the electron configuration for the neutral atom and then determine the number of electrons that are lost to form the cation. Web electron configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. Web the same rule will apply to transition metals when forming ions. The. Web therefore, the number of protons is equal to the number of electrons. As mentioned above, the electron configuration of zinc is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. A neutral chlorine atom has 17 electrons. Web the electron configuration of a neutral zinc atom in its ground state is #1s^22s^22p^63s^23p^63d^104s^2#. The fact. Web if we lose two electrons, we have a net deposited two charge. Web a neutral helium atom, with an atomic number of 2. Web the atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. This indicates that zinc has the same electronic structure as the noble gas argon (ar), followed. This element has 4 energy levels and in its outermost shell it has 2 electrons. Web to write the configuration for the zinc and the zinc ion, first we need to write the electron configuration for just zinc (zn). Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic. The electron configuration for zn +2: A neutral chlorine atom has 17 electrons. The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Web if we lose two electrons, we have a net deposited two charge. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. When writing an electron configuration, you have to write serially. _30^65zn the zinc atom has 30 protons => 30 electrons. Web to write the configuration for the zinc and the zinc ion, first we need to write the electron configuration for just zinc (zn). The fact. Electronic configuration of zinc zn: According to the electron configuration chart, electrons in an atom occupy orbitals according to their increasing energy, with each orbital having a maximum of two paired electrons with opposite spins. As mentioned above, the electron configuration of zinc is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. The electron. The atomic number of cl is 17. Web therefore, the number of protons is equal to the number of electrons. The electron configuration of a neutral zinc atom is #1s^22s^22p^63s^23p^63d^104s^2#. The ground state electron configuration of zn is [ar] 3d 10 4s 2.the orbital diagram is drawn below, which. According to the electron configuration chart, electrons in an atom occupy. 1 answer sam jul 17, 2016 #1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10# explanation: Web now in the next step, start drawing the orbital diagram for zinc. H #1s^1# he #1s^2# li #1s^2 2s^1# be #1s^2 2s^2# b #1s^2 2s^2 2p^1# c #1s^2 2s^2 2p^2# n #1s^2 2s^2 2p^3# o #1s^2. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas. Web the atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The electron configuration for the first 10 elements. Web therefore, the number of electrons in neutral atom of zinc is 30. The two electrons that we would lose to form the calcium two plus ion are these. As mentioned above, the electron configuration of zinc is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. A neutral atom has equal numbers of protons and electrons, so a neutral atom of zinc would have 30 electrons. Electronic configuration of zinc zn: Electron configuration of zinc (zn) [ar] 3d 10 4s 2: The electron configuration for zn +2: Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Chemistry electron configuration electron configuration.Draw The Electron Configuration For A Neutral Atom Of Zinc. Drawing
Atom Diagrams Electron Configurations of the Elements
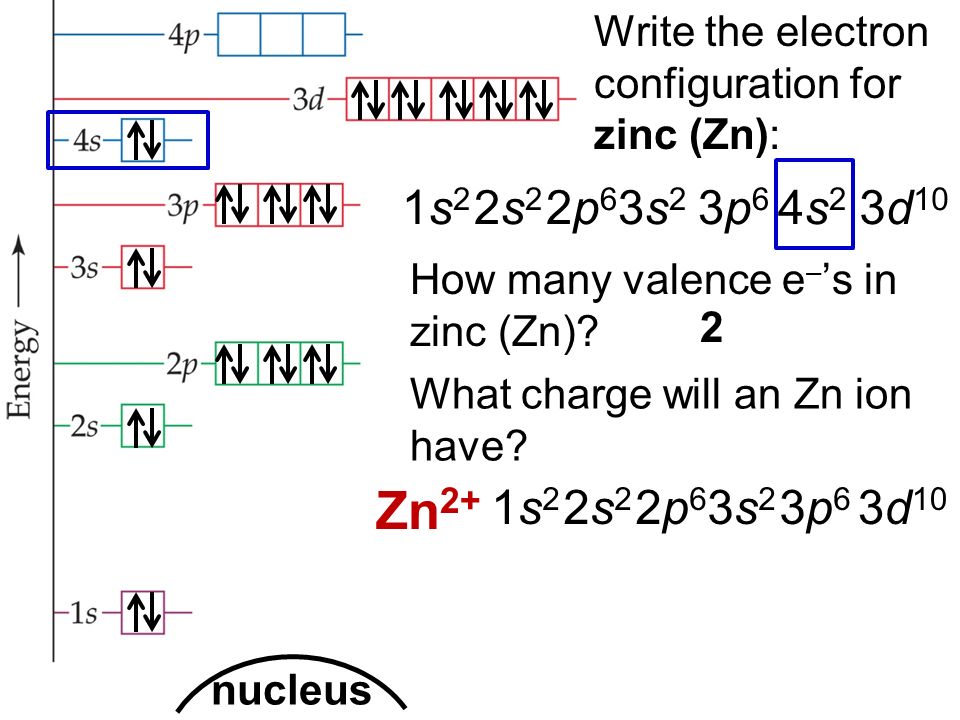
How Many Valence Electrons Does Zinc (Zn) Have?
Zinc electron configuration Stock Image C029/5029 Science Photo
Diagram Of Zinc Atom
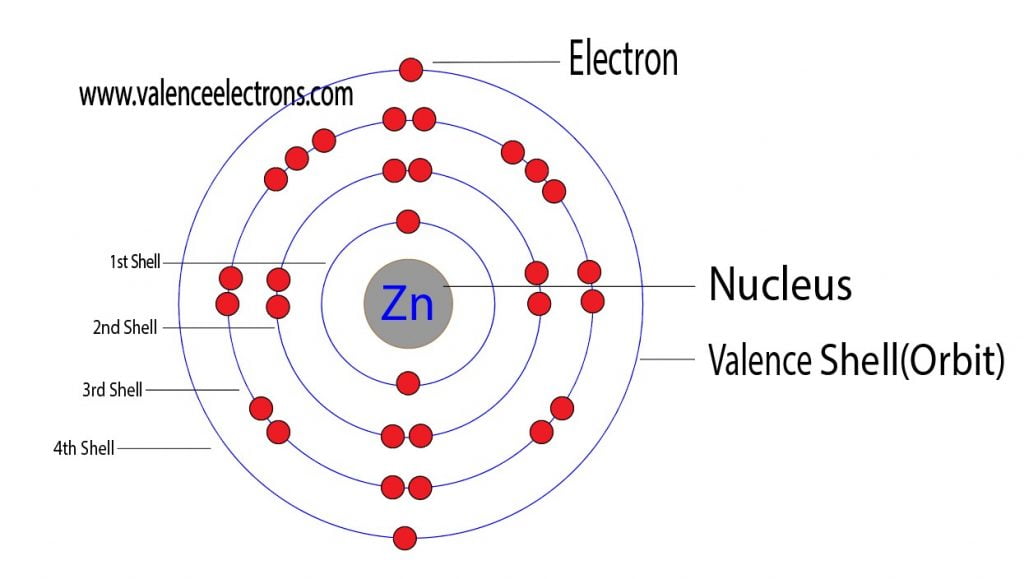
Zinc Protons Neutrons Electrons Electron Configuration
How To Find A Electron Configuration For Zinc Dynamic Periodic Table
Draw The Electron Configuration For A Neutral Atom Of Zinc. Drawing
Zinc Electron Configuration
zinc electronic configuration How to Write Zinc electronic
Zinc Has An Electron Configuration Of [Ar]3D 10 4S 2 And Is A Member Of The Group 12 Of The Periodic Table.
The Number Of The Principal Quantum Shell, N, The Letter That Designates The Orbital Type (The Subshell, L), And
Web An Atom's Ground State Electron Configuration Describes How The Electrons Have Distributed Among The Orbital Shells And Subshells.
The Electron Configuration For Calcium Two Plus Would Be The Same As The Electron Configuration For The Noble Gas Argon Here.
Related Post:

:max_bytes(150000):strip_icc()/Zinc-58b6020f3df78cdcd83d332a.jpg)